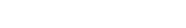Vaccination after
Vaccination after
previous SARS-CoV-2
infection
under the Feedom of Information Act 1982
Prepared by NCIRS for ATAGI COVID-19 Working Group
Thursday 27 January 2022
This document was released
FOI 3687
Document 1
1 of 16
 Current advice
Current advice
• ATAGI has decreased the time allowable for deferral of vaccination after
prior SARS-CoV-2 infection to 4 months. This is due to the increased risk
of re-infection with the Omicron variant, particularly for those who had a
Delta variant infection in 2021.
• ATAGI continues to advise that previous infection is not a contraindication
to vaccination and that vaccination can occur following recovery of acute
illness from COVID-19.
• Currently advice states that vaccination can occur following
under the Feedom of Information Act 1982
resolution of
acute illness. A precaution for all vaccination is acute illness. This may be
acute systemic signs of illness or fever. This is to avoid adverse events
(including common side effects of vaccination) in an already ill person or to
avoid attributing illness symptoms to vaccination.
• Those with prolonged symptoms of COVID-19 should be vaccinated on a
case-by-case basis.
This document was released
FOI 3687
Document 1
2 of 16
 Policy questions
Policy questions
• Any change to current advice?
• Any variation for:
• 5-11yo with prior SARS-CoV-2 infection?
• People eligible for a booster?
• Special risk groups e.g. severe immunocompromised, medically-at-risk?
• Those infected with Delta vs Omicron?
under the Feedom of Information Act 1982
This document was released
Page 3
FOI 3687
Document 1
3 of 16
 UK advice
UK advice
• As clinical deterioration can occur up to two weeks after infection, vaccination of adults and
high risk children should be deferred until clinical recovery to around four weeks after onset
of symptoms or four weeks from the first confirmed positive specimen in those who are
asymptomatic.
• This interval may be reduced in periods of high incidence or where there is concern about
vaccine effectiveness (for example a new variant).
• In younger people, protection from natural infection is likely to be high for a period of
months, and vaccination in those recently infected may increase the chance of side effects.
under the Feedom of Information Act 1982
Therefore, vaccination should ideally be deferred till at least twelve weeks from onset (or
sample date) in children and young people under 18 years who are not in high risk groups.
• This interval may be reduced to eight weeks in healthy under 18 year olds in periods of
high incidence or where there is concern about vaccine effectiveness (for example a new
variant).
https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1045288/20220105ComirnatyCovid-
19VaccineProtocolV06.00.docx#:~:text=As%20clinical%20deterioration%20can%20occur,in%20those%20who%20are%20asymptomatic.
This document was released
Page 4
FOI 3687
Document 1
4 of 16
 Other countries
USA
Other countries
USA
Defer until recovery from acute illness and completion of isolation
Israel
Those who recovered from COVID-19 can get vaccinated if at least 3 months have
passed since their date of recovery or the date of their positive result on a serologic
test.
Canada
Quebec: wait until symptoms resolve (previously 8 week interval recommended)
Ontario: wait 30 days
under the Feedom of Information Act 1982
BC: after self-isolation period and at least 10 days since onset of symptoms, or for
those without symptoms, the date of a positive test result
NZ
In a person who has had a previous SARS-CoV-2 infection, an individual is considered
fully vaccinated after two doses of mRNA-CV (or another COVID-19 vaccine,
see
section 5.8.2). In these individuals, vaccination is recommended to be given from
four weeks after recovery, or four weeks from the first confirmed positive PCR test if
asymptomatic, and when cleared to leave isolation by a clinician. This also applies to
the second dose for individuals who have SARS-CoV-2 infection after their first dose.
This document was released
Page 5
FOI 3687
Document 1
5 of 16
 Review of evidence
Review of evidence
under the Feedom of Information Act 1982
This document was released
FOI 3687
Document 1
6 of 16
 Protective immunity from infection
Protective immunity from infection
• Pre-Omicron RR of reinfection was 0.15 in previously infected vs
uninfected1
• Omicron: RR of reinfection 0.81 [95%CI: 0.73-1.00]
• Omicron had 5.41 (95% CI: 4.87-6.00) fold higher risk of reinfection
compared with Delta2
• Multiple pre-Omicron studies show prior infection and vac
under the Feedom of Information Act 1982cination provided
similar levels of protection against subsequent infection3-6
• One CDC study suggested vaccination was more effective than prior
infection7
1.
https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(21)00675-9/fulltext
2.
https://www.imperial.ac.uk/media/imperial-college/medicine/mrc-gida/2021-12-16-COVID19-Report-49.pdf
3.
https://academic.oup.com/cid/advance-article/doi/10.1093/cid/ciac022/6507165
4.
https://jamanetwork.com/journals/jama/fullarticle/2781112
This document was released
5.
https://www.medrxiv.org/content/10.1101/2021.07.03.21259976v2
6.
https://www.medrxiv.org/content/10.1101/2021.04.20.21255670v1
Page 7
7.
https://www.cdc.gov/mmwr/volumes/70/wr/mm7044e1.htm
FOI 3687
Document 1
7 of 16
 Protective immunity against Omicron from prior infection
Protective immunity against Omicron from prior infection
Shrestha et al
• >50,000 employees of Cleveland Clinic
• 7851 infections, 37% during Omicron wave
• Among previously infected, vaccination was associated with a lower risk of
COVID-19 in both
• Pre-Omicron period: HR 0.6 (95% CI 0.4 – 0.9)
under the Feedom of Information Act 1982
• Omicron period:
HR 0.36 (95% CI 0.23 – 0.57)
https://academic.oup.com/cid/advance-ar
This document was released ticle/doi/10.1093/cid/ciac022/6507165
Page 8
FOI 3687
Document 1
8 of 16

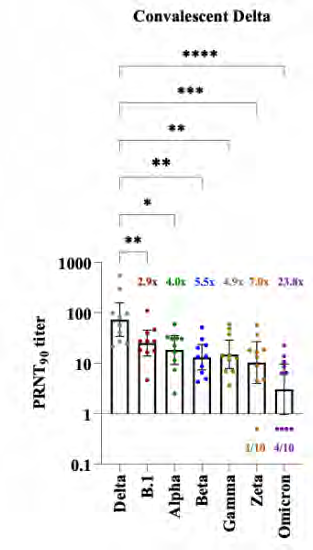
 Variant cross-
Variant cross-
neutralisation
Omicron evades
Delta-induced
immunity
104 convalescent
samples
under the Feedom of Information Act 1982
https://www.medrxiv.org/content/10.1101/2021.12.28.21268491v1.full.pdf
This document was released
Page 9
FOI 3687
Document 1
9 of 16

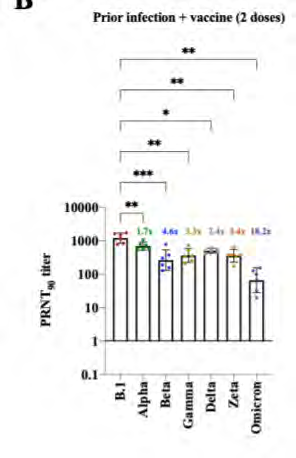
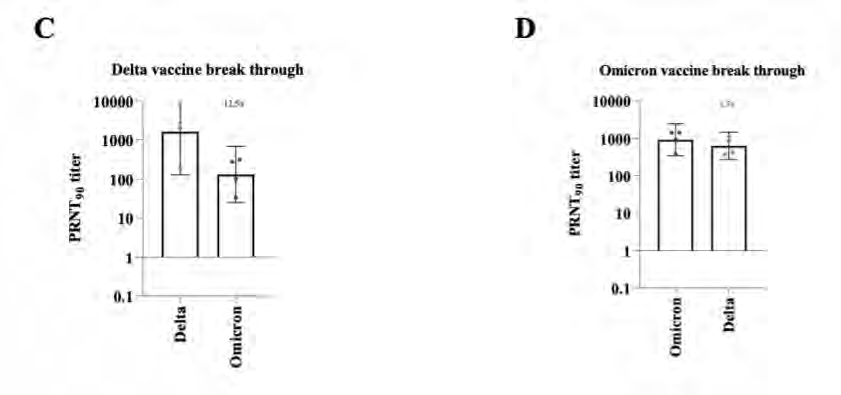 Variant cross neutralisation
Variant cross neutralisation
under the Feedom of Information Act 1982
This document was released
https://www.medrxiv.org/content/10.1101/2021.12.28.21268491v1.full.pdf
Page 10
FOI 3687
Document 1
10 of 16

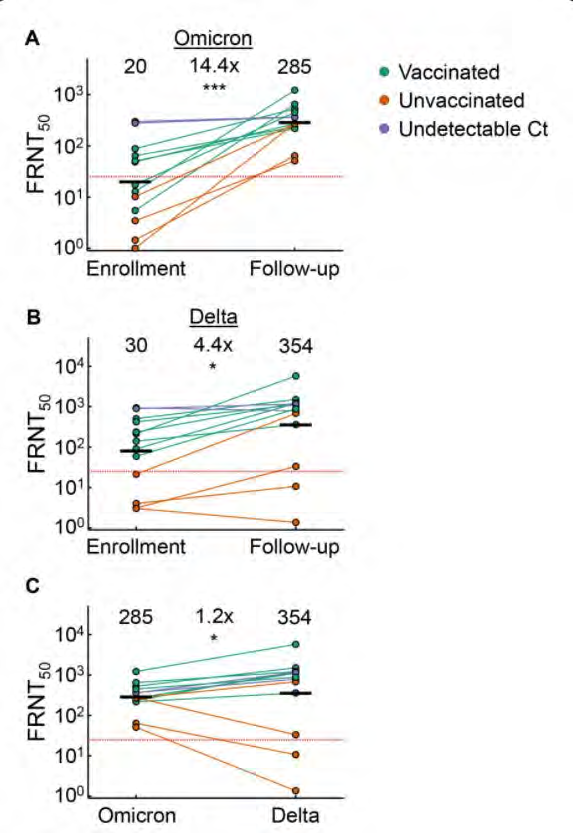 Variant cross-neutralisation
Variant cross-neutralisation
Omicron infection enhances
neutralising immunity against
Delta
15 participants infected with
Omicron in SA
under the Feedom of Information Act 1982
Neutralization of Delta
increased 4.4-fold (95% CI
2.1-9.2), from FRNT50 of 80
to 354, over 14 day
enrolment period
Khan, Khadija, Farina Karim, Sandile Cele, James Emmanuel San, Gila Lustig, Houriiyah Tegally, Mallory Bernstein et al. "Omicron infection
enhances neutralizing immunity against the Delta variant."
medRxiv (2021).
This document was released
https://www.medrxiv.org/content/10.1101/2021.12.27.21268439v1.full
Page 11
FOI 3687
Document 1
11 of 16

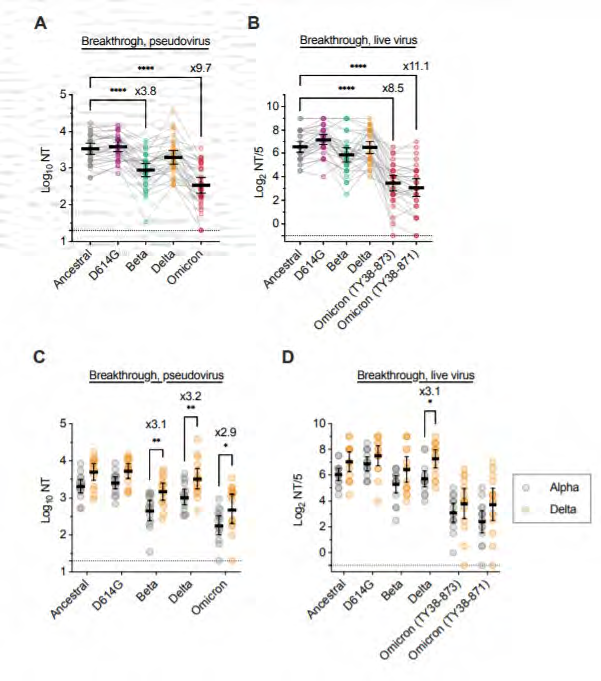 Impact of interval between
Impact of interval between
vaccination and breakthrough
infection
• Cross-neutralisation of Omicron in
mRNA-vaccinated individuals with
Alpha or Delta breakthrough
infection
under the Feedom of Information Act 1982
https://www.medrxiv.org/content/10.1101/2021.12.28.21268481v1.full.pdf
This document was released
Page 12
FOI 3687
Document 1
12 of 16

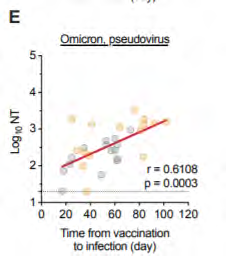
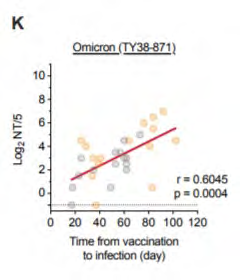
Correlation between interval
from vaccination to
breakthrough infection (with
Alpha or Delta) and
neutralisation titres against
Omicron pseudovirus (E) and
under the Feedom of Information Act 1982
live virus (K)
https://www.medrxiv.org/content/10.1101/2021.12.28.21268481v1.full.pdf
This document was released
Page 13
FOI 3687
Document 1
13 of 16
 Summary
Summary
• Omicron invades immunity induced by infection with prior variants
• Compared with prior variants, Omicron is resistant to neutralisation from
antibodies induced by prior vaccination or infection
• Better in those with longer interval between vaccination and breakthrough
infection
under the Feedom of Information Act 1982
• Conversely, Omicron induces strong neutralising activity against Delta
variant
This document was released
Page 14
FOI 3687
Document 1
14 of 16
 Current advice
Current advice
• ATAGI has decreased the time allowable for deferral of vaccination after
prior SARS-CoV-2 infection to
4 months. This is due to the increased risk
of re-infection with the Omicron variant, particularly for those who had a
Delta variant infection in 2021.
• ATAGI continues to advise that previous infection is not a contraindication
to vaccination and that vaccination can occur following recovery of acute
illness from COVID-19.
• Currently advice states that vaccination can occur following
under the Feedom of Information Act 1982
resolution of
acute illness. A precaution for all vaccination is acute illness. This may be
acute systemic signs of illness or fever. This is to avoid adverse events
(including common side effects of vaccination) in an already ill person or to
avoid attributing illness symptoms to vaccination.
• Those with prolonged symptoms of COVID-19 should be vaccinated on a
case-by-case basis.
This document was released
FOI 3687
Document 1
15 of 16
 Policy questions
Policy questions
• Any change to current advice?
• Any variation for:
• 5-11yo with prior SARS-CoV-2 infection?
• People eligible for a booster?
• Special risk groups e.g. severe immunocompromised, medically-at-risk?
• Those infected with Delta vs Omicron?
under the Feedom of Information Act 1982
This document was released
Page 16
FOI 3687
Document 1
16 of 16
Document Outline